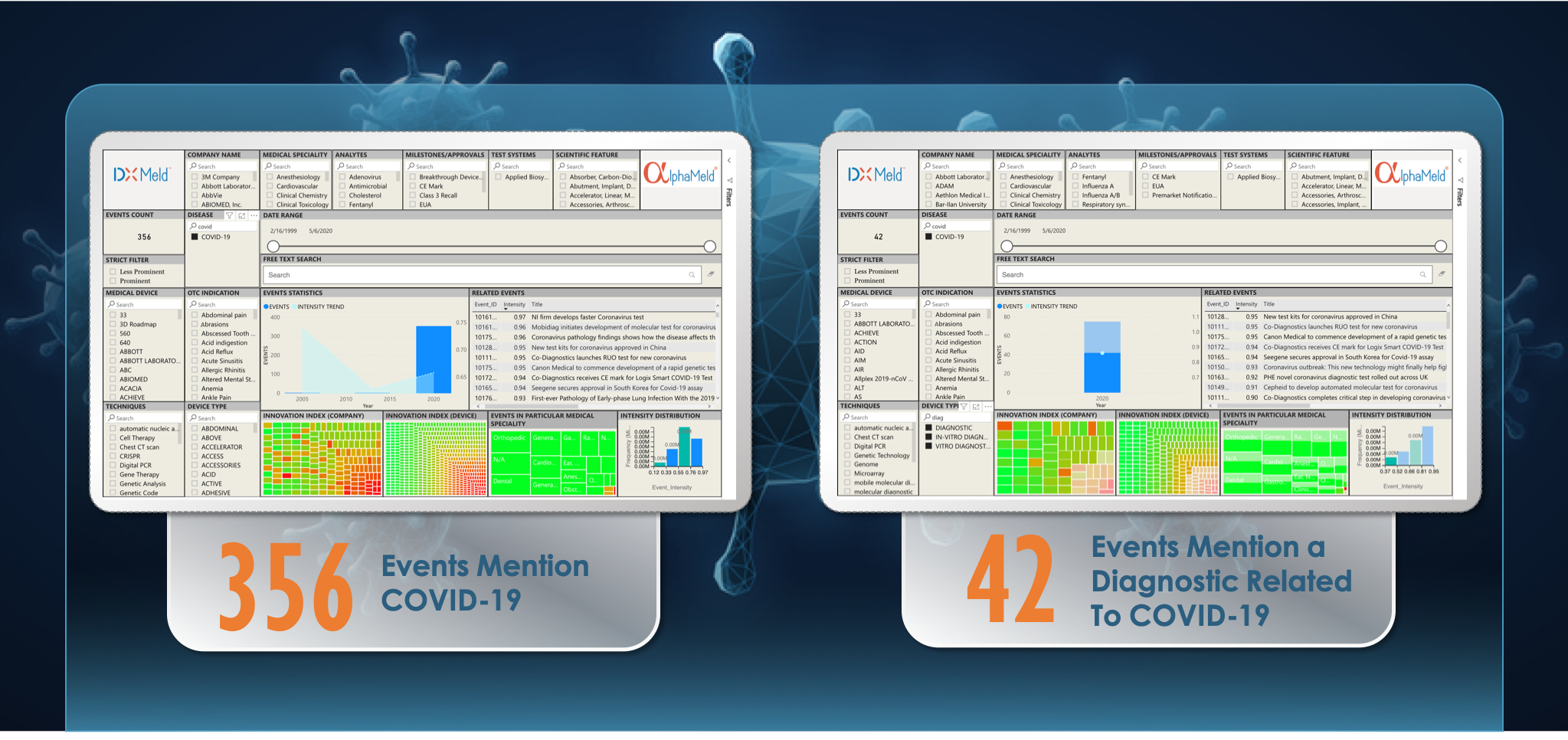
As countries scramble to address the coronavirus outbreak that started in Wuhan, China, the U.S. FDA issued a new policy to help expedite the availability of a diagnostic to achieve more testing capacity in the U.S. As the number of cases approaches the 100,000-mark, the current bottleneck remains an accurate and widely available coronavirus diagnostic test. Access to tests that can identify asymptomatic patients and the progression of the disease will play a key role in efforts to contain the epidemic and determine the course of treatment options. This will provide precious time for pharmaceutical companies to address the void of efficacious therapeutics and a vaccine option that can combat the disease.
AI technology can turbo-charge these efforts as time is limited, and accuracy is crucial. Leapfrogging current bottlenecks and getting relevant information into the appropriate hands will allow the experts to focus on what’s important. An analysis by our innovation monitoring AI-platform, DxMeld™, was performed to access global innovation in diagnostics and device development. Analyzing more than half a million data points, DxMeld™ identified the latest top-scoring tests and technologies in various stages of development, including approved, launched, CE Mark, EUA status, and in R&D. We anticipate these innovations could have a significant impact on the testing, diagnosis, and containment of COVID-19. Letting AI do the leg-work accelerates the development of tests. Delivering innovation into the hands of those developing diagnostics and treatment options will provide a timely and much-needed response to COVID-19.
Some of the diagnostic innovations highlighted by DxMeld™ are:
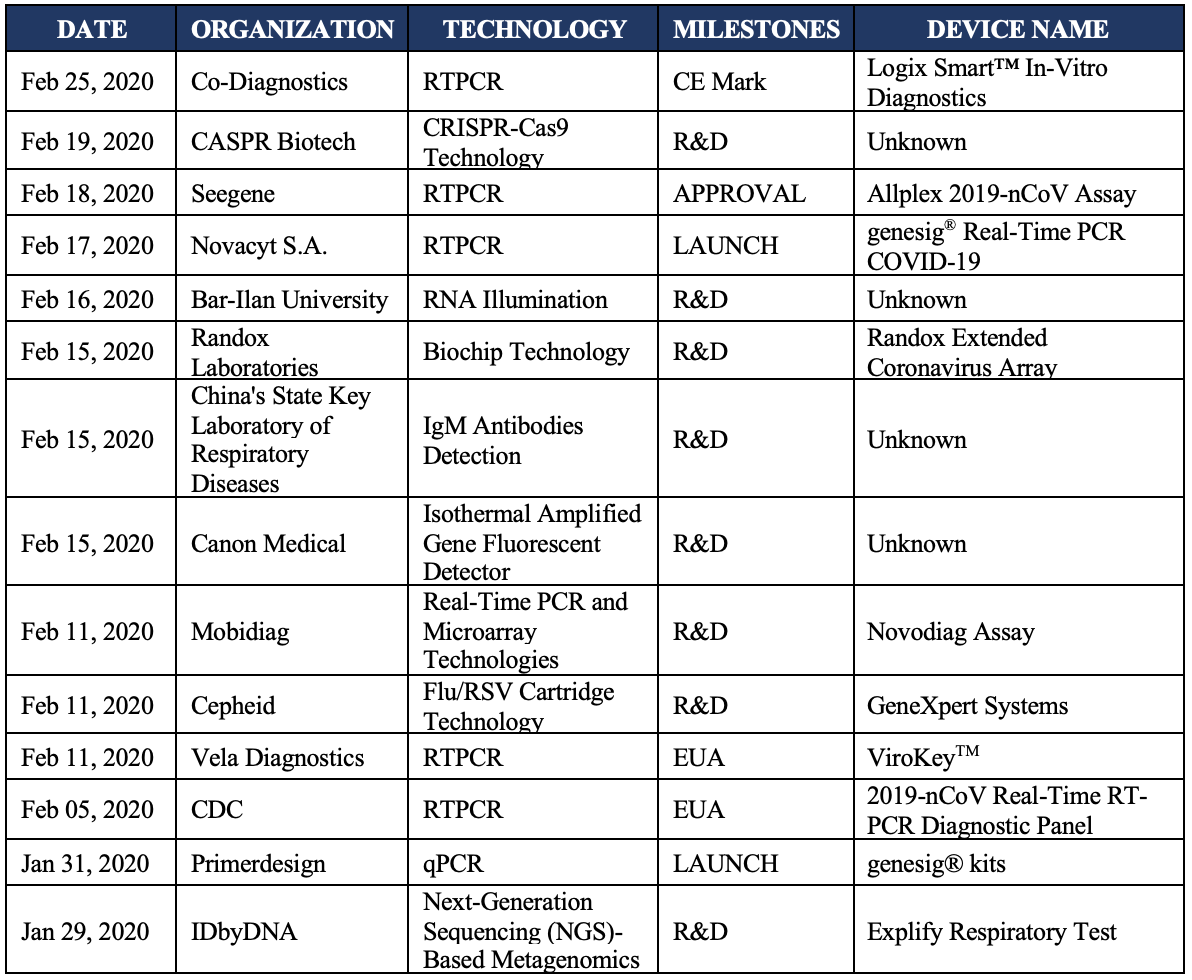
Following are some of the highlights of the above-mentioned tests:
February 25, 2020 – Co-Diagnostics Receives CE Mark for Logix Smart COVID-19 Test
The Logix Smart™ Coronavirus Disease 2019 (COVID-19) Test kit is an in vitro diagnostic test that uses patented CoPrimer™ technology for the qualitative detection of the RNA from SARS-CoV-2 coronavirus (COVID-19).
The test operates using a single step real-time reverse transcriptase-polymerase chain reaction (RT-PCR) process in lower respiratory tract specimens (e.g., bronchoalveolar lavage, sputum, tracheal aspirate), upper respiratory tract specimens (e.g., nasopharyngeal fluids, nasal swab), and serum from patients who meet the clinical criteria.
February 19, 2020 – CASPR Biotech Developed a Prototype to Quickly Diagnose Coronavirus (COVID-19)
Via CRISPR-Cas9 technology It can diagnose the virus quickly, even if the person hasn’t shown symptoms.
A person needs to provide a fluid sample (blood, urine, or a nasal swab) and in less than an hour, it will display a little black line for a positive diagnosis, and nothing for a negative result.
February 18, 2020 – Seegene Secures Approval in South Korea for Covid-19 Assay
Seegene has received approval from the South Korea Ministry of Food and Drug Safety for its novel coronavirus (Covid-19) Real-time PCR assay for emergency use.
The assay recently received the CE Mark.
Allplex 2019-nCoV Assay, which can detect three different target genes, E gene, RdRP gene, and Ngene.
Provides test results in four hours.
February 17, 2020 – Novacyt S.A.: Launch of CE-IVD Marked Novel Coronavirus Test
The genesig® Real-Time PCR COVID-19 (CE) is CE-IVD marked and intended for in vitro diagnostic use in Europe.
Rapid detection and exclusive to the COVID-19 strain.
Does not detect other related coronavirus strains.
February 16, 2020 – Novel Technology Could Significantly Reduce Coronavirus Diagnostic Time
Saliva tests can be analyzed within 15 minutes.
The high sensitivity of the platform and its ease of operation facilitate its use in point of care applications.
Dr. Danielli’s lab has developed a technology for sensitive detection of virus-specific RNA sequences by attaching the virus’ RNA to a fluorescent molecule that emits light when illuminated by a laser beam. The high sensitivity of the platform and its ease of operation facilitate its use in point of care applications where resources are limited.
February 15, 2020 – NI Firm Develops Faster Coronavirus Test
Test results differentiate between Coronavirus and nine similar viral strains
Dramatically reduces testing time for COVID 19 to between three and five hours and can also differentiate between the new strain of Coronavirus and other less-lethal respiratory infections with similar symptoms.
February 15, 2020 – China Develops COVID-19 Detection Kit that Delivers Results in 15 minutes
Can give a result in 15 minutes from one drop of the patient’s blood.
It can detect IgM antibodies, which are the first antibodies made by the body to fight a new infection, through the test of a drop of blood.
Compared with the current RT-PCR nuclei acid tests used for diagnosis, the new test kit is simpler and more efficient, with higher sensitivity and specificity.
The test and the reagents developed for COVID-19 RNA testing is based on the LAMP method developed by Eiken Chemical Co., Ltd., and is used with a compact isothermal amplified gene fluorescent detector manufactured by Canon Medical to detect the presence of a virus.
Compared to the conventional test method of real-time PCR, the LAMP method allows for the detection of the virus to be performed more easily and quickly.
February 11, 2020 – Mobidiag Initiates Development of Molecular Test for Coronavirus
Novodiag assay will leverage a fully automated ‘sample-in, result-out’ system to the viruses simultaneously in around 30 minutes.
The platform is operated without the need for highly trained personnel.
February 11, 2020 – Cepheid to Develop Automated Molecular Test for Coronavirus
GeneXpert Systems, expected to deliver point-of-care results in around 30 minutes
Once finalized, Cepheid plans to seek Emergency Use Authorization from the US Food and Drug Administration, delivering the assay worldwide.
February 11, 2020 – Mobidiag Initiates Development of Molecular Test for Coronavirus
Novodiag assay will leverage a fully automated ‘sample-in, result-out’ system to the viruses simultaneously in around 30 minutes.
The platform is operated without the need for highly trained personnel.
February 11, 2020 – Singapore Submits Coronavirus Diagnostic Test to FDA for EUA Clearance
The ViroKeyTM SA201 COVID-19 RT-PCR Test will be officially available by end of February with the facility to pre-order now.
The ViroKeyTM SA201 COVID-19 RT-PCR Test will be able to detect and differentiate the Wuhan Coronavirus from other closely related coronaviruses such as SARS and MERS.
To enable high-throughput processing of 96 samples in 4 hours, the test is configured for an automated workflow consisting of the SentosaTM SX101 instrument, in conjunction with the Applied Biosystems 7500 Fast Dx Real-Time PCR instrument (ABI 7500 Fast Dx) or the SentosaTM SA201.
February 5, 2020 – FDA Gives EUA to CDC Coronavirus Detection Test
The diagnostic is a reverse transcriptase-polymerase chain reaction (PCR) test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs.
January 31, 2020 – Primerdesign Launches Molecular Test for New Coronavirus
Genesig® kits are sold for research use only and are not licensed for diagnostic procedures.
Rapid detection of 2019-nCoV.
qPCR detection kit.
January 29, 2020 – IDbyDNA’s Explify Respiratory Test can Detect Coronavirus
US-based metagenomics technology firm IDbyDNA has announced the company’s Explify Respiratory test can detect new coronavirus (2019-nCoV).
IDbyDNA has computationally assessed the capability of the test to detect 2019-nCoV, distinguishing it from other human coronaviruses.
About DXMeld™ – An AlphaMeld® Powered Product
DxMeld™ is an innovation monitoring platform that detects the earliest signals of innovation in the field of diagnostics and devices and is part of the AlphaMeld® suite of products. The AlphaMeld® platform is powered by AI and machine learning algorithms that accelerate innovation by identifying alpha signals for targets, drugs, and healthcare products and technologies. The platform operates in real-time and recognizes these patterns in a rapidly changing and diverse data environment by engaging internal experts to personalize the definition of success and failure for an organization or vertical market. The platform eliminates dependence on a time-series and uses industry-aware scoring algorithms that are customized and further strengthened by incorporating continuous feedback through machine learning. AlphaMeld® is trained to amplify human expertise to enable robust decision-making tailored to the needs of multiple stakeholders within an organization.
Please contact us at akant@inveniai.com akant@inveniai.com to access this analysis and for more information on diagnostic innovation for COVID-19